Abstract
Background: Peripheral T cell lymphomas (PTCL) are a highly aggressive and heterogeneous group of non-Hodgkin lymphomas that carry a poor prognosis. Treatment paradigms tailored to their biology, have yet to emerge, though many of the approved drugs like histone deacetylase inhibitors and pralatrexate, exhibit interesting lineage specific activity. Tolinapant is a potent, non-peptidomimetic antagonist of cIAP1/2 and XIAP. In an ongoing Phase 2 trial (NCT02503423), tolinapant has shown activity in patients with highly pre-treated peripheral and cutaneous T-cell lymphoma. Hypomethylating agents (HMAs) have also shown clinical responses across the diversity of PTCL, with some suggestion that PTCL of T-follicular helper phenotype exhibit marked sensitivity. Both HMAs and IAP antagonists show immunomodulatory anti-cancer potential in pre-clinical studies. We have also shown that decitabine and tolinapant as single-agents induced necroptosis in mouse TCL cell lines with the activation of the RIPK1/RIPK3/MLKL necroptosis pathway. In addition, re-expression of RIPK3 in CT26 cells increased lytic cell death on treatment with tolinapant indicating its essential role in the necroptosis pathway.
Objectives: 1) Characterize the single-agent activity of tolinapant in a range of TCL lines; 2) Determine the synergy of tolinapant in combination with drugs active against PTCL (romidepsin, pralatrexate) and HMAs (azacytidine-AZA; decitabine-DAC), and 3) Define the role of necroptosis in the mechanism of synergy.
Methods: A panel of 10 human TCL lines were tested in proliferation assays (CellTiterGlo) for sensitivity to tolinapant in the presence or absence of 10 ng/ml of TNFa. For combination studies, each drug was tested at the IC10, IC20 and IC40 concentrations. Synergy coefficient was calculated using the Excess over Bliss (EOB) model with Synergy Finder. Additionally, the on-target effects of the drugs were measured by analyzing levels of the IAPs, DNMTs and key apoptosis and necroptosis markers, like PARP and RIPK3, respectively, by Western blot.
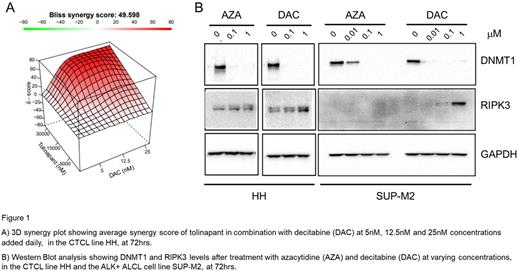
Results: TCL lines demonstrated a range of sensitivities to tolinapant with the most sensitive cell line, ALK+ ALCL SUP-M2, having an IC50 as low as 20 nM ± 1 nM while a resistant CTCL cell line HH had an IC50 of over 20 µM. In combination experiments, tolinapant plus romidepsin was synergistic in the SUP-M2 cell line (Avg. EOB score 13.3) and additive in the HH cell line (Avg. EOB score 7.9). Tolinapant plus pralatrexate was not synergistic in ether SUP-M2 (Avg. EOB score -1.06) or another resistant CTCL cell line H9 (Avg. EOB score 0.6). However, in both SUP-M2 and HH, tolinapant plus AZA or DAC displayed a high degree of synergy, with an average EOB score of 50.9 and 49.8 with AZA and 22.6 and 31.3 with DAC in HH and SUP-M2, respectively. Of note, synergy was also achieved, in the resistant cell line HH cell line, when concentrations 10 fold lower of AZA (Avg. EOB score 61.2) or 100 fold lower of DAC (Avg. EOB score 49.6; Fig 1A) were added daily. In the cell line SUP-M2, synergy was only seen, with DAC (Avg. EOB score 10.5) and not AZA (Avg. EOB score 2.1) at the lower concentrations added daily. The combination of tolinapant and the HMAs led to a decrease in the levels of cIAPs and DNMTs in both TCL lines, demonstrating on-target activity of tolinapant and the HMAs, respectively. In addition, both AZA and DAC exhibited a concentration dependent increase in the levels of RIPK3 up to 3-4 fold and 6-8 fold respectively, at 72hrs in the HH cells. In SUP-M2 cells, only DAC increased the levels of RIPK3 at 72 hrs (Fig 1B).
Conclusion:In vitro, combinations of tolinapant with romidepsin or pralatrexate displayed very little to no synergy. In contrast, tolinapant in combination with the HMAs, AZA and DAC, displayed a very high degree of synergy even at very low concentrations of both HMA drugs. The HMAs were able to re-express components of the necroptosis pathway, as shown by the concentration dependent increase in RIPK3. Likewise, tolinapant has been shown to induce necroptosis in in vivo models of TCL (Ferrari et al, Blood Adv. 5, 2021). Thus, activation of the necroptosis pathway, is a possible mechanism for the high degree of synergy displayed when HMAs are used in combination with tolinapant in the TCL lines in vitro. These data provided the rationale for a phase 1-2 study of the combination of tolinapant and oral decitabine/cedazuridine treatment in relapsed/refractory PTCL (NCT05403450).
Disclosures
Manavalan:Astex: Research Funding. Ward:Astex: Current Employment. Smyth:Astex Pharmaceuticals, Inc.: Current Employment. Sims:Astex: Current Employment. Taylor:Astex Pharmaceuticals, Inc.: Current Employment. Feith:Recludix Pharma: Research Funding; AstraZeneca: Research Funding; Kymera Therapeutics: Honoraria. Loughran:Dren Bio, Inc: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Keystone Nano: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Prime Genomics: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Recludix Pharma: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Kymera Therapeutics: Honoraria. O'Connor:TG Therapeutics: Current Employment, Current equity holder in publicly-traded company. Marchi:Merck: Research Funding; Celgene/BMS: Research Funding; Astex Pharmaceuticals: Research Funding; Kyowa Kirin: Honoraria; NomoCan Pharmaceuticals: Research Funding; Myeloid Therapeutics: Ended employment in the past 24 months, Membership on an entity's Board of Directors or advisory committees; University of virginia: Patents & Royalties: 3062/170 PROV; Daiichi Sankyo: Other: Participation at advisory board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal